Specific Heat Capacity Of Sand
Well-nigh Sand
Sand is a granular material composed of finely divided rock and mineral particles. The composition of sand varies, depending on the local rock sources and weather condition, but the most common elective of sand in inland continental settings and non-tropical coastal settings is silica (silicon dioxide, or SiO2), usually in the course of quartz. Silica is one of the near circuitous and most abundant families of materials, existing as a compound of several minerals and as synthetic product.
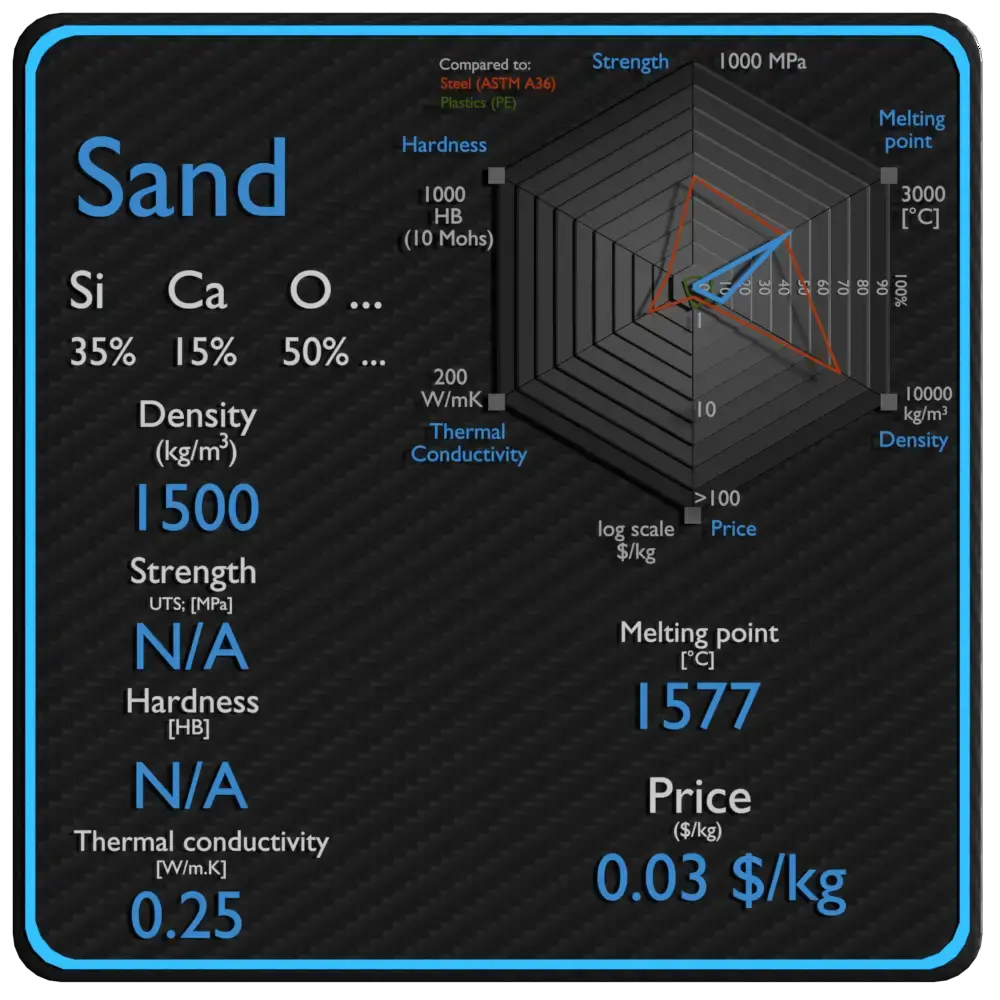
Summary
| Name | Sand |
| Stage at STP | solid |
| Density | 1500 kg/m3 |
| Ultimate Tensile Strength | N/A |
| Yield Strength | Due north/A |
| Immature's Modulus of Elasticity | N/A |
| Brinell Hardness | North/A |
| Melting Point | 1577 °C |
| Thermal Conductivity | 0.25 Due west/mK |
| Rut Capacity | 830 J/g G |
| Price | 0.03 $/kg |
Density of Sand
Typical densities of various substances are at atmospheric pressure.Density is defined as themass per unit volume. It is anintensive property, which is mathematically defined as mass divided by volume: ρ = grand/V
In words, the density (ρ) of a substance is the total mass (m) of that substance divided by the full volume (5) occupied by that substance. The standard SI unit of measurement iskilograms per cubic meter (kg/m3 ). The Standard English unit ispounds mass per cubic foot (lbm/ft3 ).
Density of Sand is 1500 kg/thousand3.
Example: Density
Calculate the height of a cube fabricated of Sand, which weighs one metric ton.
Solution:
Density is defined equally themass per unit volume. It is mathematically divers as mass divided by volume: ρ = m/V
As the book of a cube is the third power of its sides (V = athree), the height of this cube tin can be calculated:
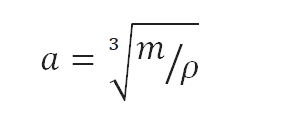
The height of this cube is and so a = 0.874 m.
Density of Materials
Thermal Properties of Sand
Sand – Melting Betoken
Melting point of Sand is 1577 °C.
Note that, these points are associated with the standard atmospheric pressure. In general,melting is aphase change of a substance from the solid to the liquid phase. Themelting signal of a substance is the temperature at which this phase change occurs. Themelting bespeaktoo defines a condition in which the solid and liquid tin can exist in equilibrium. For diverse chemic compounds and alloys, it is hard to ascertain the melting point, since they are commonly a mixture of diverse chemic elements.
Sand – Thermal Electrical conductivity
Thermal conductivity of Sand is 0.25 W/(m·K).
The estrus transfer characteristics of a solid cloth are measured by a property called thethermal electrical conductivity, thou (or λ), measured inWestward/one thousand.Thou. It is a measure of a substance's ability to transfer heat through a material past conduction. Annotation thatFourier'south law applies for all matter, regardless of its land (solid, liquid, or gas), therefore, it is besides defined for liquids and gases.
Thethermal conductivity of well-nigh liquids and solids varies with temperature. For vapors, it also depends upon pressure. In full general:
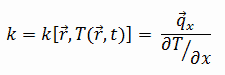
Most materials are very almost homogeneous, therefore nosotros can usually write k = k (T) . Like definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = one thousand.
Sand – Specific Heat
Specific heat of Sand is 830 J/g Chiliad.
Specific heat, or specific heat chapters,is a property related to internal free energy that is very important in thermodynamics. Theintensive backdropcfive and cp are defined for pure, simple compressible substances as fractional derivatives of theinternal energyu(T, v) andenthalpyh(T, p) , respectively:
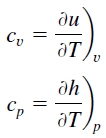
where the subscriptsfive andp denote the variables held fixed during differentiation. The propertiescvandcp are referred to asspecific heats(orheat capacities) because under certain special weather they relate the temperature alter of a organization to the amount of energy added by oestrus transfer. Their SI units areJ/kg G orJ/mol K.
Instance: Rut transfer calculation
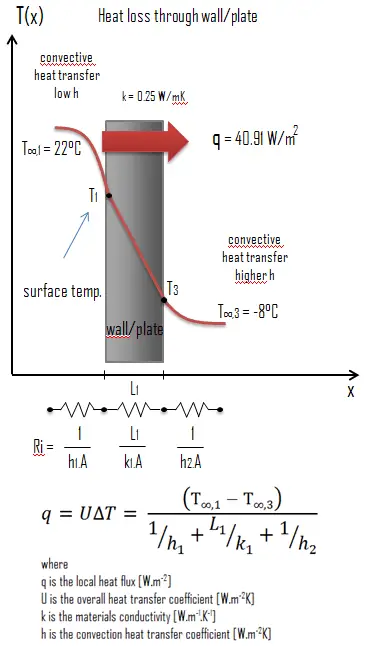 Thermal conductivity is defined as the amount of heat (in watts) transferred through a square surface area of textile of given thickness (in metres) due to a difference in temperature. The lower the thermal conductivity of the textile the greater the material's ability to resist heat transfer.
Thermal conductivity is defined as the amount of heat (in watts) transferred through a square surface area of textile of given thickness (in metres) due to a difference in temperature. The lower the thermal conductivity of the textile the greater the material's ability to resist heat transfer.
Calculate the charge per unit ofheat flux through a wall iii thou x 10 m in area (A = xxx mtwo). The wall is 15 cm thick (Lone) and it is made of Sand with the thermal conductivity of ki = 0.25 W/m.Grand (poor thermal insulator). Assume that, the indoor and the outdoor temperatures are 22°C and -eight°C, and theconvection heat transfer coefficients on the inner and the outer sides are h1 = 10 W/chiliadiiK and h2 = 30 Westward/one thousand2K, respectively. Annotation that, these convection coefficients strongly depend particularly on ambience and interior atmospheric condition (current of air, humidity, etc.).
Calculate the heat flux (oestrus loss) through this wall.
Solution:
As was written, many of the heat transfer processes involve composite systems and even involve a combination of bothconduction andconvection. With these composite systems, information technology is often convenient to work with an overall rut transfer coefficient ,known every bit aU-factor. The U-factor is defined by an expression coordinating toNewton's law of cooling:

Theoverall heat transfer coefficient is related to the total thermal resistance and depends on the geometry of the problem.
Bold one-dimensional rut transfer through the plane wall and disregarding radiations, theoverall heat transfer coefficient tin be calculated as:
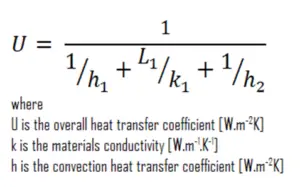
The overall oestrus transfer coefficientis then: U = 1 / (1/10 + 0.15/0.25 + 1/30) = 1.36 W/grand2K
The heat flux can exist and so calculated simply every bit: q = ane.36 [Westward/mtwoOne thousand] x 30 [Thousand] = forty.91 Westward/m2
The total heat loss through this wall will be: qloss= q . A = forty.91 [Westward/mtwo] x 30 [one thousand2] = 1227.27 West
Melting Point of Materials

Thermal Conductivity of Materials
Heat Chapters of Materials
Forcefulness of Materials

Elasticity of Materials
Hardness of Materials
Specific Heat Capacity Of Sand,
Source: https://material-properties.org/sand-density-heat-capacity-thermal-conductivity/
Posted by: cogswellreacquink.blogspot.com


0 Response to "Specific Heat Capacity Of Sand"
Post a Comment